We want to see the immune system in action...
...right down it its chemistry.
We ask questions such as:
How do cells of the immune system respond to inflammation? How do the location and the timing of stimulation affect the response?
What is the best way to build a model of the immune system to replicate immunity outside the body? Answering this question would reveal the mechanisms of
How does the immune response vary between individuals, and can we predict that variation to enable precision medicine for immunological diseases?
With these questions in mind, we build the microfluidic devices, chemical assays, and computational models that are needed to answer them. Ultimately, our work will create new tools to understand how the immune system is organized. We and others will use these tools to make progress towards therapies for diseases such as multiple sclerosis, Alzheimer's disease, and solid tumors.
We have several lines of ongoing research:
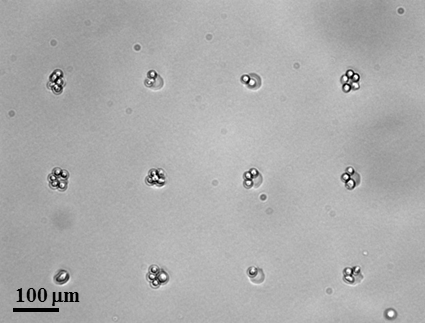
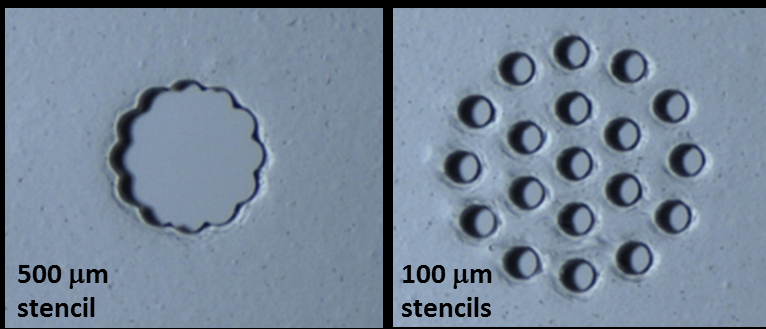
1. We are developing a spatially organized in vitro model of a human lymph node, also called a lymph-node on a chip (organ-on-chip system) or a “microphysiological system.” In 2025 we shared a preprint describing the results of this model, which predicts antibody responses (an early type called IgM) from naive T cells and B cells. This NIH U01-funded project integrates biomaterials design, analytical chemistry, and micropatterned 3D cell culture, microfluidic environmental control, and deep expertise from our immunology collaborators. We continue to grow the biomimicry of the model, with a goal of allowing it to respond to vaccination or infection. In this effort, we are part of the NIH TissueChip Consortium and aspire to eventually integrate the lymph node with other organ-on-chip models, for full multi-organ immunity, and to integrate the diversity of human immune responses into the lymph-node chip.
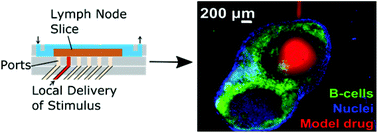
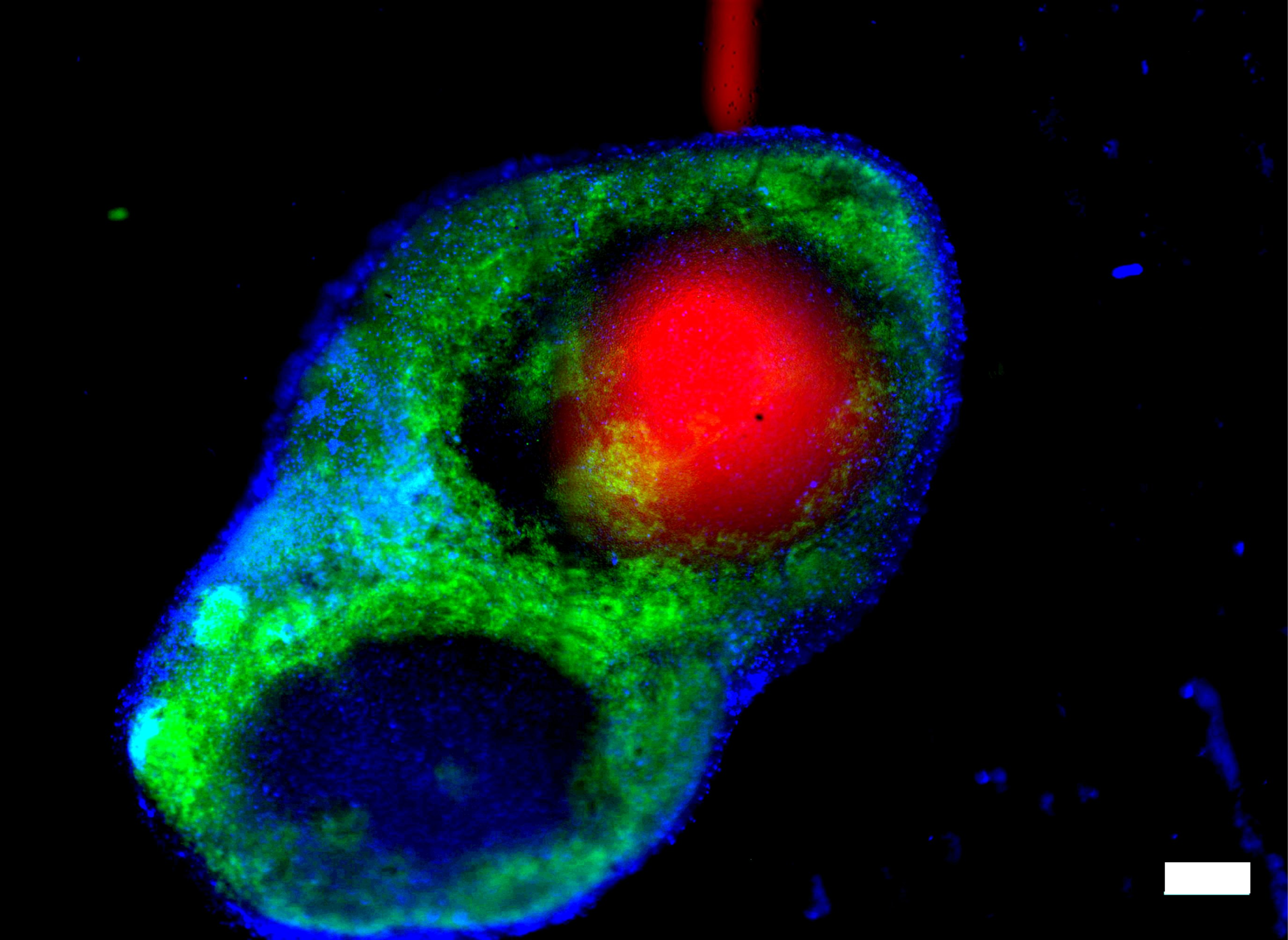
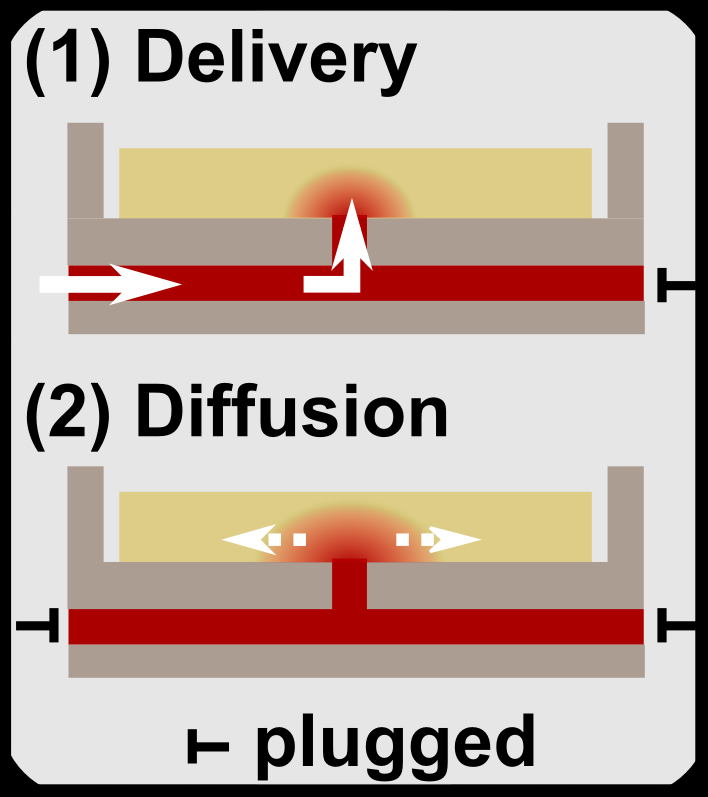
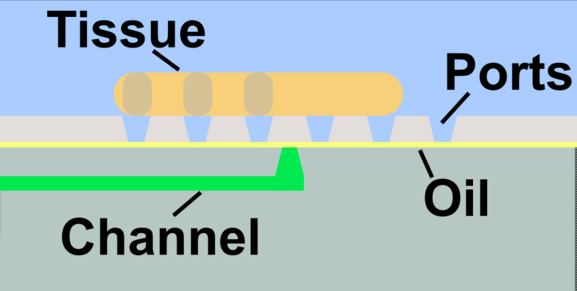
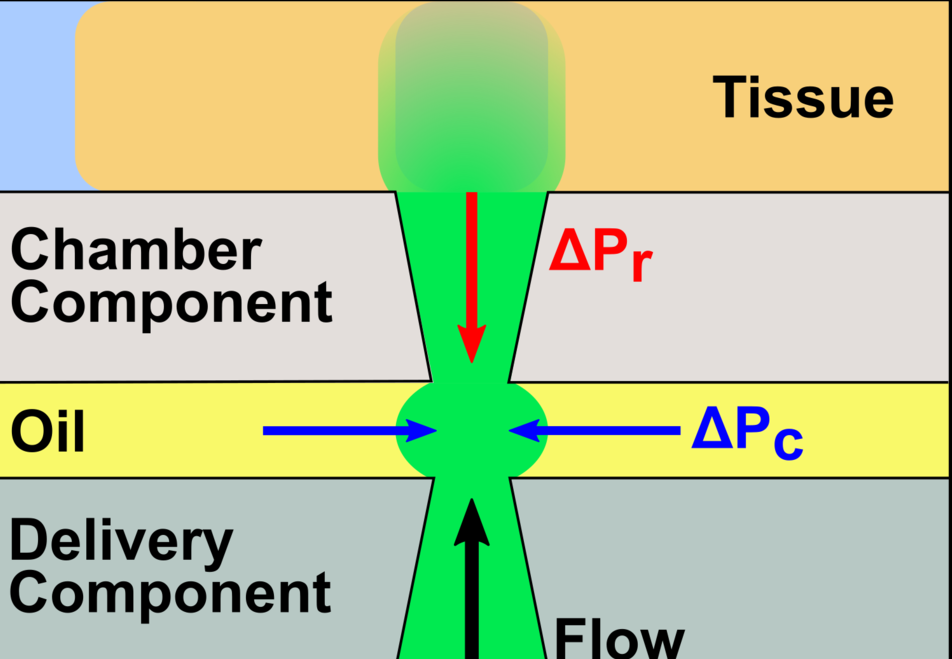
2. We develop develop and utilize microfluidic and organ-on-chip platforms for ex vivo analyses of live lymphoid tissues. For example, we developed devices for local drug delivery to lymph node tissue, which may be used to study molecular events in the tissue and to determine where vaccines and immunotherapies should be targeted. More recently, we are focused on building multi-organ models of immunity, with the ultimate goal of understanding how tumors condition nearby lymph nodes (early prototype here), how lymph nodes respond to vaccination (prototype here), and lymph nodes are altered during autoimmunity and neurodegeneration. This work combines cutting edge microfabrication, such as digital light processing 3D printing, along with device design to replicate immunological endpoints.
3. Working with all of these tissues and complex 3D cultures requires that we can analyze the responses in a spatially resolved manner. We design new biochemical assays to visualize the chemistry in living tissue. We are happy to share our method for “live immunostaining” of cells and structures in live tissue explants. We have assays in the works to detect secreted proteins. Earlier we developed methods to map out oxygen consumption and glucose consumption in living tissue, enabling visualization of metabolic activity during the immune response.
3. We established that live ex vivo slices of murine lymph node tissues as a model of immunity, and now are translating this to work with samples of surgically discarded human lymphoid tissue, such as tonsils after tonsilectomy. We are now applying this unique culture model to learn about the response to vaccination, tumor metastasis to lymph nodes, and other systems. We are happy to spread this useful system to other laboratories, and indeed have already done so. Ask us for more information if interested.
Applications: Chronic Inflammatory Disease and Tumor Immunology
Currently, attention in the lab is focused on developing tools to understand the mechanisms of chronic inflammation in the context of autoimmune disease and cancer immunology. Chronic inflammation arises when the immune system gets caught in an ongoing state of activation towards a target that cannot be cleared -- for example, against proteins in the joints (rheumatoid arthritis), in the gut (inflammatory bowel disorder), in the myelin that protects neurons in the brain (multiple sclerosis), or in a solid tumor. This state is poorly understood, in part because it is difficult to reproduce it in vitro, and in part because it is driven by a complex set of chemical signals -- cytokines and chemokines -- that have been difficult to quantify over time and with sufficient spatial resolution.
Our core toolbox includes:
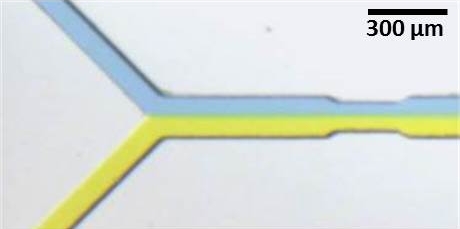
1. Microfluidics and organs-on-chip
At their simplest, microfluidic devices are miniature plumbing systems built from channels that are tens to hundreds of micrometers (microns) wide. Because most cells from humans and other mammals are approximately 5 - 20 microns in diameter, microfluidic channels are ideally sized to manipulate and stimulate samples of cells and tissues.
We build microfluidic devices from glass, soft or hard polymers (plastics), or biomaterials such as agarose and gelatin. In all cases, the chemistry on the surface of the device can be modified as needed. For example, surfaces can be made hydrophilic or hydrophobic, cell-adhesive, or protein-repellent. The device can also be used to deliver controlled gradients of signalling molecules.
In this area, we have a major effort in the field of organs-on-chip. We are developing novel methods to replicate immunity outside of the body.
2. Bioanalytical chemistry
Cells in tissues communicate with one another by releasing proteins and small molecules that travel to their neighbor (or farther!) to carry a message. If we as scientists want to "listen in," most chemical approaches measure the molecules after they have been collected in a fluid -- cell culture media, serum, urine, etc. This approach relinquishes all information about where each molecule came from. In our lab, we specialize in detecting proteins and other biological signals at their source, in living tissue.
3. Live fluorescence imaging
Seeing immunity in space and time means imaging it while it happens. We make extensive use of various types of microscopy at both the tissue scale and cellular scale to observe the location and behavior of cells in our tissues.
4. Biomaterials and 3D cell culture
For bottom-up models of the lymph node, we start by suspending cells in a 3D culture, using biomaterials as a matrix or scaffold. In collaboration with colleagues at UVA and Virginia Tech, we have begun exploring how immune function is best modeled in these materials.
Streaming on-demand:
Prof. Pompano @ Labroots Virtual Event
First aired May 30, 2019
Now available on YouTube
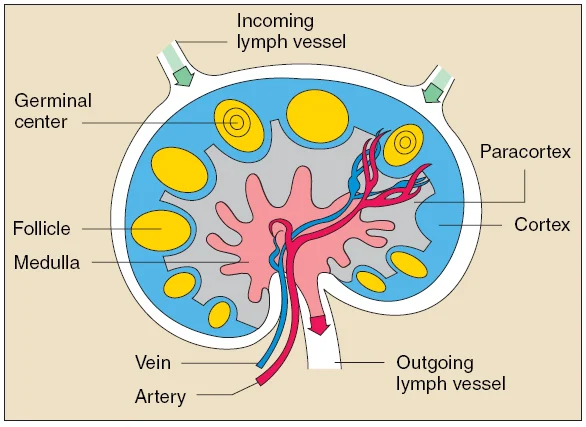
Lymph nodes are highly structured organs. T cells and dendritic cells are localized to the paracortex, while B cells are found in follicles and germinal centers near the edge of the node. Cells and proteins traffic in and out of the node through blood vessels and lymphatic vessels.
Image reproduced from the NIAID at the NIH.